As explained on cellular metabolism 1, during catabolism, larger molecules are broken into smaller ones and the released energy is immediately packaged into energized biomolecules that are used for moment-to-moment energy transfers in the cell.
ATP introduced these energized biomolecules and discussed the all-around energy-mover "ATP". This page covers NADPH and its role in anabolism (making larger molecules out of smaller ones).
ATP is used in everything from cellular "housekeeping" to the movements of muscle cells to the molecule-building of anabolism. However, the chemical reactions of anabolism often require something that ATP doesn't provide called "reducing agents".
1. 2Na + Cl2
→
2. 2Na+ + 2e- + Cl2
→
3. 2Na+ + 2Cl-
→
4. 2NaCl
This is a simple redox reaction.
In (2.), the 2Na has given up
two electrons to become 2Na+.
In (3.), the Cl2 has picked up
two electrons
to become 2Cl-.
In (4.), the 2Na+ and the 2Cl-
have bonded to
form 2NaCl.
"Reduction" is when a molecule, atom or ion loses at least some control over electron(s). "Oxidation" is when a molecule, atom or ion gains at least some control over electrons.
For either to happen, the reduction and oxidation need to happen
at the same time and
together they are called an
"oxidation-reduction reaction" ("redox reactions" is
the nickname their friends use).
In the example on the right, electrons are lost by sodium atoms and picked up by chlorine atoms.
However, you can also have an oxidation-reduction reaction where electrons are not completely transferred and one substance just has less of a "share" of its electron(s) than it used to (this is what happens in redox reactions that break and form covalent bonds - see oxidation-reduction).
In these sorts of chemical reactions, a substance that gives up an electron (gets itself "oxidized) is called a "reducing agent" and one that gains an electron (gets itself "reduced") is called the "oxidizing agent".
NADPH molecules are created in catabolism when a negative hydride anion is bonded to a molecule of NADP+. A "hydride anion" (H-) is a hydrogen atom with an extra electron (two e- instead of one e-) and therefore a negative charge.
When used as a reducing agent, each NADPH molecule gives up the hydride anion (H-), providing two electrons (2 e-) to help move the reaction forward. In the process, a NADP+ molecule is also released. (1)
Reducing agents are important in anabolism. The formation of many of the molecules your cells need (for example, all fatty acids) relies on NADPH as a reducing agent to help drive the needed oxidation-reduction reactions forward.
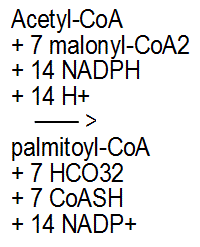
The chemical reaction on the left is of the reaction that creates a molecule of the fatty acid palmitate. As you can see, before the reaction there are 14 NADPH molecules and afterwards 14 NADP+ molecules. (2)
Just as ADP molecules can (with the help of energy and a phosphate group) be turned into new ADT molecules, NADP+ molecules can (also with the help of energy and a hydride anion) be turned back into NADPH molecules. And so - at least at a cellular level - you recycle.
If this page has been a help to you
please recommend it with Google+ below:
1 My information on how NADPH is made and used comes the second edition of the textbook Biochemistry by Garrett and Grisham, currently (6/20/2011) available online at
Heidi.
(1) How NADPH is produced: This is introduced in the subsection "NADPH Provides the Reducing Power for Anabolic Processes" of
section "18.2 • Metabolism Consists of Catabolism (Degradative Pathways) and Anabolism (Biosynthetic Pathways)". The
relevant quotation is: "NADPH is generated when NADP+ is reduced with electrons in the form of hydride ions."
(2) More Details of How NADPH is produced and used: The details about how NADPH involves the transfer of a hydride anion
comes from subsection "Nicotinic Acid and the Nicotinamide Coenzymes" of "18.4 • Nutrition." The relevant passage comes from
a discussion of the role of NADP in catabolism (not discussed on this webpage) and NADPH in "reductive (biosynthetic) pathways": "These reactions involve direct transfer of
hydride anion either to NAD(P)+ or from NAD(P)H. The enzymes that facilitate such transfers are thus known as
dehydrogenases. The hydride anion contains two electrons, and thus NAD+ and NADP+ act exclusively as two-electron carriers."
2 This equation for the synthesis of palmitate was taken from the textbook (Biochemistry) discussed and linked-to in footnote #1; It was taken from the subsection "Fatty Acid Biosynthesis Depends on the Reductive Power of NADPH" in the section "25.1 × The Fatty Acid Biosynthesis and Degradation Pathways Are Different". In the subsection "Formation of Malonyl-CoA Activates Acetate Units for Fatty Acid Synthesis" of the same section, it is stated that ATP is used to form the Malonyl-CoA.