|
|
ENERGY CHANGES MAKE THINGS HAPPEN
|
|
|
|
|
| A simple definition of energy; Energy changes and transformations make things happen; Some examples of energy changes making things happen; Stored Solar Energy is the fuel for most living organisms and human made machines; a little bit about Energy and Heat Flow; Some different types of Energy; the First Law of Thermodynamics and other intriguing things you should know about energy; a final example of monkey's, bananas, and energy conversions. |
|
|
|
|
|
For anything to happen there always has to be an energy conversion or change.
An example of Some of the Energy Changes that have to happen in order for a person to Jump are given below: |
 It always starts in the sun, where matter is changed into electromagnetic energy and zips out into space. The energy a person uses to jump always comes first from the sun. It always starts in the sun, where matter is changed into electromagnetic energy and zips out into space. The energy a person uses to jump always comes first from the sun. |
A tiny bit of the sun's energy falls onto earth. On earth some of the solar energy is changed by photosynthesis into chemical energy stored in the carbohydrate molecules in plant cells. |

A human eats a plant. Or a human eats an animal that ate a plant. The chemical energy stored in the plant (or animal) cells is moved into the cells of the human's body. All of the body processes, like digestion, pumping blood, breathing, are powered by cells converting the stored chemical energy into work and heat, in a process called respiration. Respiration takes place in every cell in your body. |

Inside the muscle cells of the human (or any animal), the chemical energy is transformed (changed) into mechanical work and heat. The muscle contracts, the legs push, and the body leaps into the air. Some of the chemical energy has now been changed into the kenetic energy of a body flying up into the air. The rest of the original chemical energy has been used to raise the temperature of your jumping body. If you keep jumping for long you'll get pretty hot. |
|
|
|
|
Introduction to Energy Changes -
Or The Changes that Make Things Change
 Energy is that "certain something" inside stuff (or matter to be more precise) that makes everything happen. When something or somebody moves or jumps or falls or explodes or breathes or thinks or dances or does anything, it's because energy is being transformed. Energy is that "certain something" inside stuff (or matter to be more precise) that makes everything happen. When something or somebody moves or jumps or falls or explodes or breathes or thinks or dances or does anything, it's because energy is being transformed.
 Energy is
being transformed
or changed from one type of energy to another, or, in the case of heat flow, from one place to another place.
Energy is
being transformed
or changed from one type of energy to another, or, in the case of heat flow, from one place to another place.
There are some things about the way energy always behaves that everybody (yes everybody) ought to know about. This is the section that will introduce you to the basics of energy behavior. You will discover here that you are very interested in energy.
"This is good stuff," you will say to yourself over and over, as you read through these pages.
"I never knew how much I loved thermodynamics," you will add as a happy afterthought.
We start with the stories of a dancin' fool, a cd player, a locomotive, and some of the interesting energy changes that make music, dancing, freight trains, and tree climbing monkeys, possible. |
|
|
 |
 |
|
 |
 |
 |
|
|
|
|
|
|

|
|
|
|
|
 |
 |
 |
 |
|
|
|
|
|
Some Things You Should Know About Energy:
1) For anything to happen there has to be a transfer or change or conversion of Energy.
- A transfer from one type of energy to another type - like from chemical to mechanical, or potential to kinetic.
- Or a transfer of energy from one place or area to another place or area (an engineer would say from one system to another system).

- Energy always moves from a "higher concentration" of energy to a "lower concentration" of energy. (What the hec does that mean? We'll tell you in the next installment).
2) There are two ways that energy can be transferred - by work or by heat flow.
In this section we are talking about heat flow. Work will come later. (But guess what! Down at the itsy-bitsy micro level of molecules and atoms, it's all really work! Really! It's all just work! Heat flow is sort of an illusion. It is the average effects of billions and billions of atoms and molecules!)
3) Energy never appears from nowhere or disappears into nothingness. This reality is called the First Law of Thermodynamics. Text books often say it this way, "Energy is neither created nor destroyed", or "Energy is Conserved". (Those kinds of definitions usually only make sense to you if you already understand it. So, if this is new to you, keep reading for some easy to follow examples).
FURTHER NOTE: What about the 2nd Law of Thermodynamics, you ask? Aren't I going to talk about that? Yup. But not here. Well, we'll mention it here and there a little, like in Item 1) above - the stuff on higher to lower concentrations of energy is part of it. |

The diesel powered locomotives rumble and roar and a mile long train pulls out of Denver. Thousands of tons of coal in more than 100 coal cars is on its way to San Antonio. Inside the coal are millions of BTU's of stored chemical energy waiting quietly for the chance to be converted into heat and electrical energy at a power plant in Texas.
We take such human engineering wonders for granted nowadays. It is interesting to consider that the locomotives are powered by stored solar energy, just like the jumping humans above, and leaping monkeys below. Just to keep you thinking about such things, let's consider some of the energy changes necessary to power a locomotive.
A summary below, more detail in coming pages. |

Once again (and again and again and again) it starts with that great fusion furnace in the sky we call the sun. But in this case it started a much longer time ago. Just as described above, some of the solar energy that landed on green plants was converted into plant material and carbohydrates.
This time, however, the plants weren't eaten. This time they died, got buried, and spent a good long while deep under the earth being slowly turned into petroleum. |
 The crude oil that is pumped out of the ground is taken to an oil refinery where it is turned into all kinds of hydrocarbon fuels like gasoline, kerosene, jet fuel, diesel fuel, and more. The crude oil that is pumped out of the ground is taken to an oil refinery where it is turned into all kinds of hydrocarbon fuels like gasoline, kerosene, jet fuel, diesel fuel, and more. |
Now the energy changes in the engine can start.
In the fuel tank the diesel fuel is stored chemical energy waiting to happen. Sometimes people call energy waiting to happen potential energy. |
 Squirt diesel fuel into a diesel engine cylinder and suddenly the fuel is burning so fast and so hot we call it an explosion. The potential chemical energy that was so quietly waiting in the diesel fuel is turned very quickly into heat. The heated air and combustion products in the cylinder expand, the piston is pushed down by the expanding air pressure, the crankshaft turns, and mechanical work is done. Chemical energy was transformed into mechanical work. Ta Da! Squirt diesel fuel into a diesel engine cylinder and suddenly the fuel is burning so fast and so hot we call it an explosion. The potential chemical energy that was so quietly waiting in the diesel fuel is turned very quickly into heat. The heated air and combustion products in the cylinder expand, the piston is pushed down by the expanding air pressure, the crankshaft turns, and mechanical work is done. Chemical energy was transformed into mechanical work. Ta Da! |
On a locomotive the diesel engine crankshaft turns an alternator which converts mechanical energy into electrical energy. The electrical energy goes to giant traction motors which convert the electrical energy back into the rotating mechanical torque that turns the wheels.

The turning wheels push the locomotive forward. Now the mechanical energy becomes the kinetic energy of motion of the whole coal train. Don't get in front of it, because it will require a lot of brake friction turned into heat to stop this behemoth.
As explained elsewhere, all of this energy eventually becomes low grade energy from friction and from heat rejected by the engine. This raises the surrounding air temperature a little bit. Eventually the energy is radiated back out into space. If it didn't the earth would keep getting warmer and warmer, and we would all find things pretty uncomfortable. |
(see the 2nd Law of Thermo Page)
In one liter of diesel fuel there are approximately 39,887 KJ (37,807 Btu) of potential chemical energy waiting to be turned into heat. If all this energy could be turned into work (which it can't) it would be enough energy to lift a 1000 kg weight 4070 meters (13,056 feet) above the earth.
 The best locomotive engines can turn only about 44% of the fuel they burn into useful mechanical work. Another 33%, or so, goes out the stack as very hot exhaust gas. The remaining 23% goes mostly into the cooling water and engine oil. All of the fuel energy, even the part that's turned into useful work, eventually ends up heating the air, then radiating out into space. The best locomotive engines can turn only about 44% of the fuel they burn into useful mechanical work. Another 33%, or so, goes out the stack as very hot exhaust gas. The remaining 23% goes mostly into the cooling water and engine oil. All of the fuel energy, even the part that's turned into useful work, eventually ends up heating the air, then radiating out into space. |
Isn't Energy the Ability to do Work?
Yes.
You will learn from text books and other sources that the definition of energy is "the ability to do work". That is the standard definition. But we think the definition we are using, "that certain something inside stuff with the ability to make things happen", is more helpful as an introduction to the concept of energy.
For anything to happen work has to be done on something. Even in the case of heat flow, which in classical thermodynamics is not considered a work process, work is being done on molecules or atoms.
At some level work is always being done when something happens. So we really are saying the same thing. |
|
|
|
|
Whole Lot of Changes Goin' On and On and On...
THERE HAS TO BE AN ENERGY CHANGE TO MAKE THINGS HAPPEN!
Put a cd in your portable player, put on the headphones and flip the switch.
Chemical energy that was "waiting around", doing nothing much but "sitting" in the battery being potential, suddenly starts turning into electrical energy. The new electrical energy, formerly known as chemical, zips through some wires to the electric motor that spins the disc. Now the energy that used to be electrical has become the mechanical energy of the spinning disc.
An electrical signal from sensors that "read" the spinning disc, travels through a wire to the little speakers in the headphones. In the headphones, the electrical energy that carried the signal, now becomes sound energy (sound waves).
Where the Voices Inside Your Head Come From
Inside your head, sound energy in your ears is changed into electrical and chemical signals that travel to a part of your brain where those signals are turned into a sensation we call music.
Your brain sends electrical signals to muscles in your legs, arms, face, and other places. Inside the muscle cells chemical energy is stored in glucose molecules (see Introduction to Photosynthesis). The signals from your brain tell your muscles to contract. The muscles convert the stored chemical energy into mechanical work, and you start to move. (The process in plant and animal cells that converts glucose into work and heat is called respiration). The energy in the glucose came from a plant or animal you ate. By now, you should know from where the plant got the energy.

You use a bunch of face muscles to smile. You move arm and leg muscles in a coordinated manner (some people move in a more coordinated manner than others). You strut and gyrate to the music. Some people call this dancing. You're busy thinking how cool you are while billions of cells in your body are busy coverting chemical energy into work and heat.
You start to sing off key and too loudly because of the headphones. Singing requires using the diaphram muscles below your stomach to force air through the larnyx in your neck. Once again chemical energy in the muscles is changed into mechanical energy (and heat). Then the mechanical energy in the contracting muscles is changed into mechanical pressure and flow energy (kinetic) of the air being pushed out of your lungs.
The larnyx changes the pressure and flow energy into sound energy which travels as sound waves through the air and bothers many of the easily annoyed people within earshot who don't seem to appreciate your awesome talent. The sound waves in their ears are also changed into electrical signals inside their brains, but somehow they aren't as happy about it as you are. So they don't use muscle cells to smile.
Epilogue to the CD-Dancer Story:
The First Law of Thermodynamics tells us that all of the previously potential energy that came out of the battery and out of the dancer's muscle cells doesn't go away to the mysterious dimension of nothingness. It has to still be around somewhere. Where is that somewhere? It all ends up as a slight increase in the energy of air molecules.
Yes, it actually heats up the air a little bit. A little bit warmed the battery, then flowed into the air. A little bit warmed the wires, then flowed into the air. The spinning cd and motor did work on some air molecules, causing them to zip around faster and transfer their newly increased kinetic energy to still more air molecules. The cells in your body gave off heat which flowed out of your body into the air. Your dancing limbs also pushed billions of air molecules out of the way. All of that heat and motion was eventually transferred to the air, slightly increasing the energy of billions of air molecules. After that it was radiated out into space where it will help to warm up the universe an itsy-bitsy, teensy-weensy (yellow polka-dotted) little bit.
 That's really what happens. Energy comes from the sun, gets used awhile, then gets sent back out into space. At the end of its usefullness it is what mechanical engineers often call low-grade energy. When energy becomes low-grade it is no longer in a form that is very useful to life (except maybe to help keep things a little warmer). Having worn out its welcome, the hapless energy radiates back out to the cold heat sink of outer space. Luckily for us a new fresh dose of "high-grade" energy from the sun radiates into the earth everyday. That's really what happens. Energy comes from the sun, gets used awhile, then gets sent back out into space. At the end of its usefullness it is what mechanical engineers often call low-grade energy. When energy becomes low-grade it is no longer in a form that is very useful to life (except maybe to help keep things a little warmer). Having worn out its welcome, the hapless energy radiates back out to the cold heat sink of outer space. Luckily for us a new fresh dose of "high-grade" energy from the sun radiates into the earth everyday.
The Numero Uno Law of Thermodynamics says that, over a certain period of time, the energy that is radiated back into space plus the energy that stays behind on earth, has to exactly equal the energy that comes in from the sun. If the energy coming into the earth from the sun does not equal the energy radiated back out into space, then the earth's average temperature will change over time. If more energy comes in than goes out, the earth's average temperature would start to go up, and would keep warming up until the energy in and out is again balanced. If more energy goes out than comes in, then the earth's average temperature will get cooler.
This basic law of thermodynamics is why many scientists are worried about the "green house effect". They are concerned that increasing CO2 and other green house gasses will trap in more energy over time and raise the average temperature of the earth. Many scientists (but not all - we have to be fair) believe this is already happening.
One More Time
"There Has to be a Change or Transfer of Energy"
We keep saying it. Nothing can happen in this wondrous world without energy. Energy is that certain something inside "stuff" that makes things happen.
| But it isn't just energy. Energy can sit around doing nothing for a long time, like in a battery or a tank of diesel fuel. Nothing will happen until that "waiting" potential energy gets changed into another form of energy. |
|
|
 |
| It's energy moving from one place to another place, or energy being changed from one type of energy into another type of energy, that makes things happen. |
|
|
A flashlight converts the chemical energy stored in batteries into light and heat. Most of the energy is converted to heat. Only a small percentage of the original energy in the battery is converted into light energy. |
 |
 |
 |
 |
Is There A Definition?
This "energy-changing-making-things-happen" concept is often not made quite clear enough in books and websites. Thermodynamics is not so much the study of energy, as it is the study of energy being changed. Energy that is not "happening" is only a concept of what might be. Potential energy for example is really just a mathmatical concept describing something that can happen.
Think I'm being vague and evasive about energy? Listen to what Richard Feynman, one of the best known physicists of the twentieth century said, "It is important to realize that in physics today, we have no knowledge of what energy is. We do not have a picture that energy comes in little blobs of a definite amount".
Here's another one from a guy named David Rose (MIT engineering professor famous for his work in fusion, energy, and nuclear engineering): "Energy is an abstract concept invented by physical scientists in the nineteenth century to describe quantitatively a wide variety of natural phenomena".
Now do you understand?
That's why in the Mysterious Everything page, I emphasize that what scientists and engineers really do is study, describe, and predict, how energy behaves when it is in transition.
So learning about energy is learning how it behaves, what it does and what it does not. We can use our knowledge of how energy behaves to build machines and power plants and to better understand the processes of nature, but we can't really tell you what the heck it is.
I like it that way. Science is full of mysteries and unanswered questions. A person can be properly scientific and skeptical and still have a sense of wonder. If you're a person who has to have all the answers, then you probably won't like science.
Scientists have to spend a lot of time wondering and asking questions. Science still has many more questions than answers - lots and lots to learn. Lots of stuff for good science "detectives" to find out.
|
|
Thermodynamics is more than just a fun-to-say word. It is also a field of science and engineering. All this stuff about energy conversions and heat and work is stuff that you will study when you study thermodyanamics.
But you can't truly be a thermodynamic know-a-lot unless you know the following:
The word "thermo" comes from some old time language and stands for heat or energy (depending on who you talk to), while "dynamics" stands for something like movement or motion or change. So together they sort of mean "energy-changes" or "energy- movement".
It's energy changes we're interested in. Energy wouldn't be very interesing if it never changed. Nothing would be very interesting because nothing would ever happen. |
|
|
|
In the next section we discuss the concept of heat flow more thoroughly.
An understanding of heat and heat flow is necessary to fully appreciate the fascinating ways animals and engineers control the flow of heat. Some fascinating examples of such things will be coming to these pages soon.
Don't forget the cute diagram below on energy conversions in monkeys. |
|
|
|
|
|
|
|
monkey
| In the final example below, a monkey finds an abundance of tasty stored solar energy in the carbohydrate molecules of a banana. Though we don't show it in the diagram below, it is worth remembering that the first energy conversion begins in the sun, where matter is converted to energy in a nuclear fusion reaction during which Hydrogen atoms are changed into Helium atoms. A by-product of this process is tremendous amounts of a type of energy called electromagnetic radiation (visible light, x-rays, ultraviolet light, infrared, gamma-rays, micro-waves, and radio waves) that pours out of the sun into space . Some of this former-matter-turned-into-energy falls on earth. Down here we call it solar energy. |
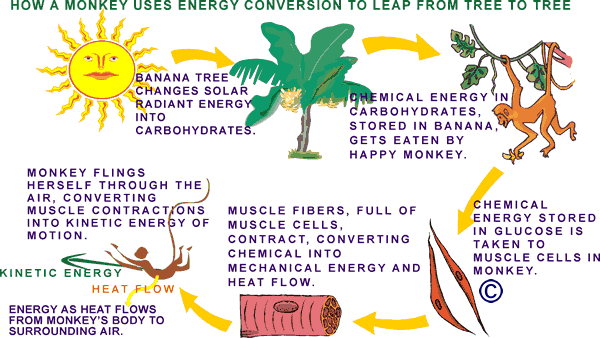
Every cell in the monkey's body (and ours) is constantly converting the stored solar energy in glucose into work and heat. The work is used to carry on cell processes like growing, reproducing, moving molecules around, and getting rid of waste. The heat is a byproduct of the fuel "burning" process. Heat is always given off when fuel is burned, whether it is in a diesel engine or an animal cell. In an animal the heat can be used to help keep its body at a certain warm temperature. Sometimes our bodies make too much heat (especially if we are dancing to loud music) and we have to do things like sweat or pant (if we are dogs) or fan our big heat exchanger ears (if we are elephants) to try to cool down.
All of the heat flows eventually into the surrounding air. The quantity of total energy has not, and will not, change. It has just moved to different forms and different places. |
|
 |
 |
 |
 |
 |
|
|
|
|
|
|
|
|
|
 |
 |
 |
 |
 |
|
|

